We understand that with pharmaceuticals, every element needs to go through a licensing procedure. However, there are some changes that blister packaging has to undergo when making the switch from manual to automation.
How blister packaging works
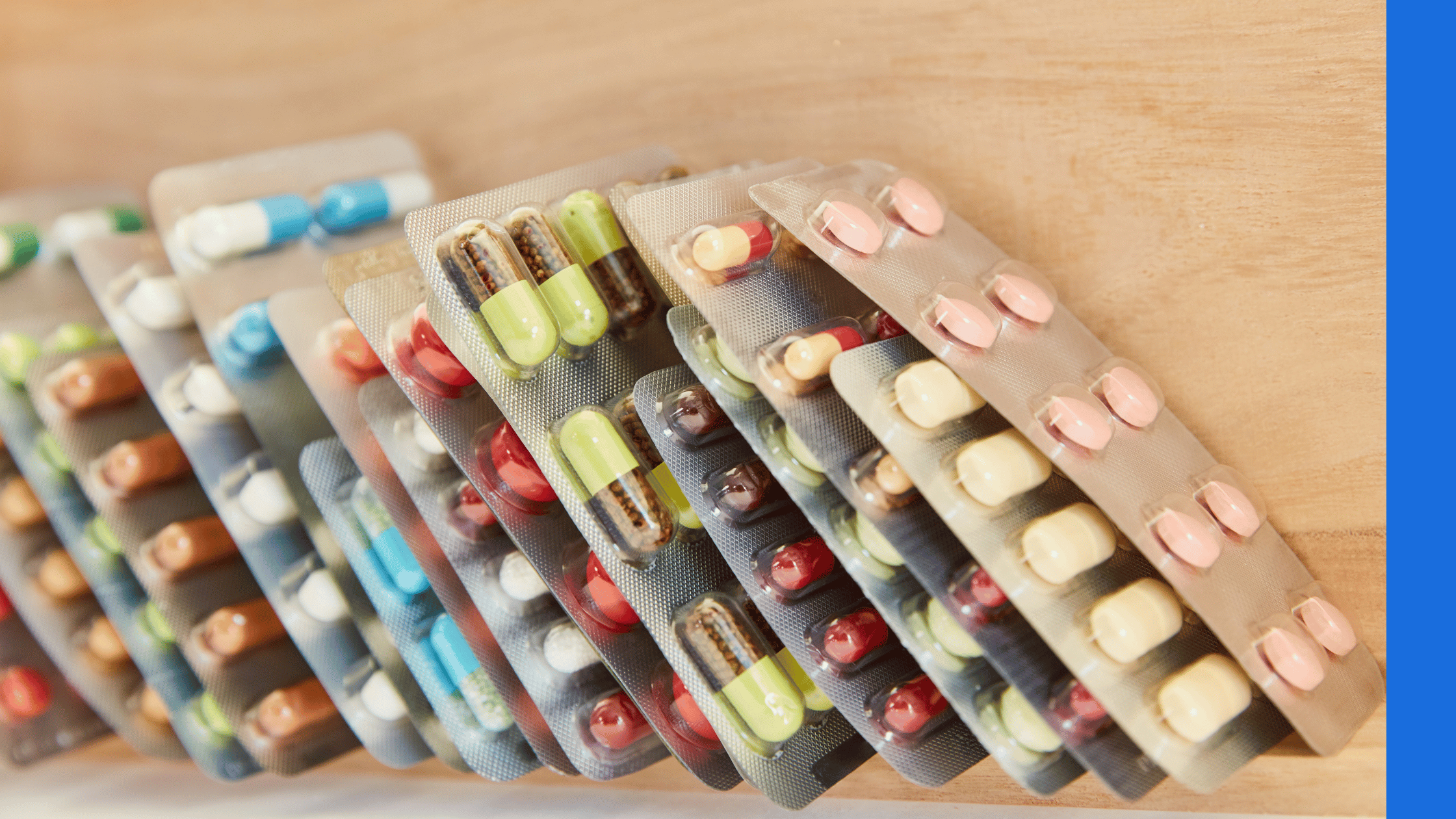
Blister packaging can be used for tablets, capsules, syringes, contact lenses and more. This type of packaging protects the product against moisture, heat, light and other contaminants. To create the packaging, a blister is formed by the machinery in plastic, aluminium, paper or other medium – this can be achieved through vacuum, thermoforming or cold forming. This is then filled with the product, sealed, and can be perforated or cut before moving on to be packaged in an outer box.
Pharmaceutical packaging standards
Government agency, the Medicines & Healthcare products Regulatory Agency (MHRA) is the body which approves all labelling and packaging information of pharmaceuticals sold in the UK. They provide guidance on best practice on the labelling and packaging of medicines.
Making the switch to automated blister packaging
The way that your blister packaging is currently formed, the layout, and the materials – these elements all need to be considered as sometimes it’s not possible for them to stay exactly the same when switching to automated packaging.
While there is some versatility with layout, blister packaging machines make, fill and seal the packs at such speed, all elements need to be optimised for best performance – and sometimes this means changing your packaging.
For pharmaceutical companies, we would always recommend building extra time into your automation schedule to allow for packaging changes. It can take over six months to get even slightly amended packaging approved.
Understanding automation
Pharma Machinery offer a range of user-friendly blister packaging machinery, and can advise you on how the packaging will need to be set up to automate it efficiently. We’ll work with you to match you with the best machine for your needs. Contact us today for a free consultation.